NOGGO S18 - EMRISK
DEVELOPMENT OF A RISK PREDICTION MODEL FOR NAUSEA AND VOMITING DURING CHEMOTHERAPY
What is being investigated in this study?
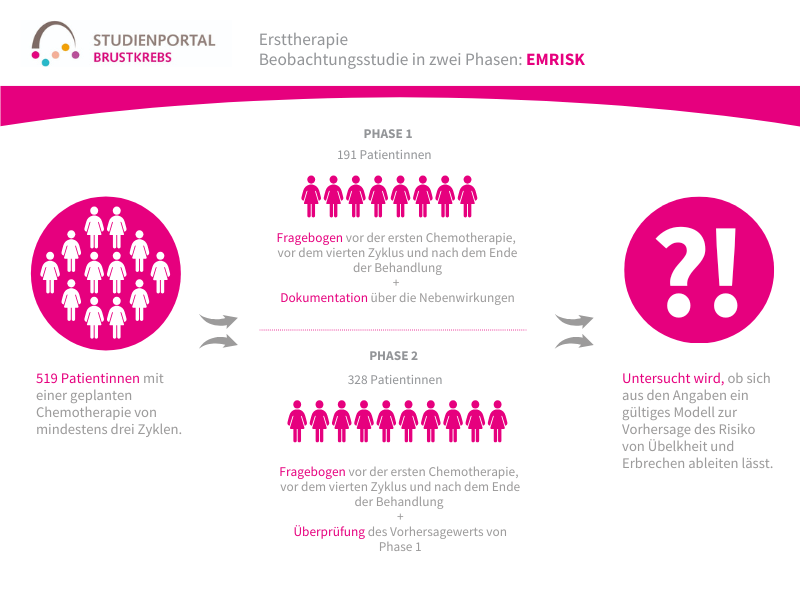
The EMRISK study is a two-phase observational study in which patients are asked about their history and risk factors for nausea and vomiting and about the actual occurrence of nausea and vomiting during chemotherapy. The study aims to determine whether this information can be used to derive a valid model for predicting the risk of nausea and vomiting.
What is the goal of the study?
This study aims to develop a model to predict a patient’s risk of nausea and vomiting during chemotherapy.
How is the study conducted?
The study is being conducted on a total of 519 patients. In the first phase, 191 patients fill out a questionnaire before their first chemotherapy session, which provides information about their personal risk factors for nausea and vomiting. During chemotherapy treatment, a diary is provided to document when and how severely nausea and vomiting actually occur.
The aim is to identify correlations between risk factors and the development of nausea and vomiting in order to develop a model that calculates the personal risk for each individual patient. Before the first chemotherapy session, before the fourth cycle and after the end of treatment, patients are asked to complete the QLQ-C30 questionnaire about their quality of life. In the second phase, this model will be applied to 328 patients before their treatment in order to verify its predictive value.
The first phase of the study has already been successfully completed.
Are there risks?
No, EMRISK is an observational study. The patients participating in the study would receive the same treatment if they were not participating in the study. In this respect, participation in the study does not entail any risks.
Participation Requirements
Women aged 18 years and older who
- have been diagnosed with primary breast cancer,
- are scheduled to undergo at least three cycles of adjuvant and neoadjuvant chemotherapy (as standard therapy),
- are willing and able to complete the questionnaires and keep the diary,
- and have provided written informed consent are eligible to participate in this study.
Where can I participate in this study?
Further information on participating centres can be found here:
https://studienportal-brustkrebs.de/en/map-of-germany/
This study is supported by: