ADAPTlate Study
Personalized therapy study in patients with hormone receptor-positive, HER2 receptor-negative early-stage breast cancer who have a clinically or genomically high risk of late recurrence
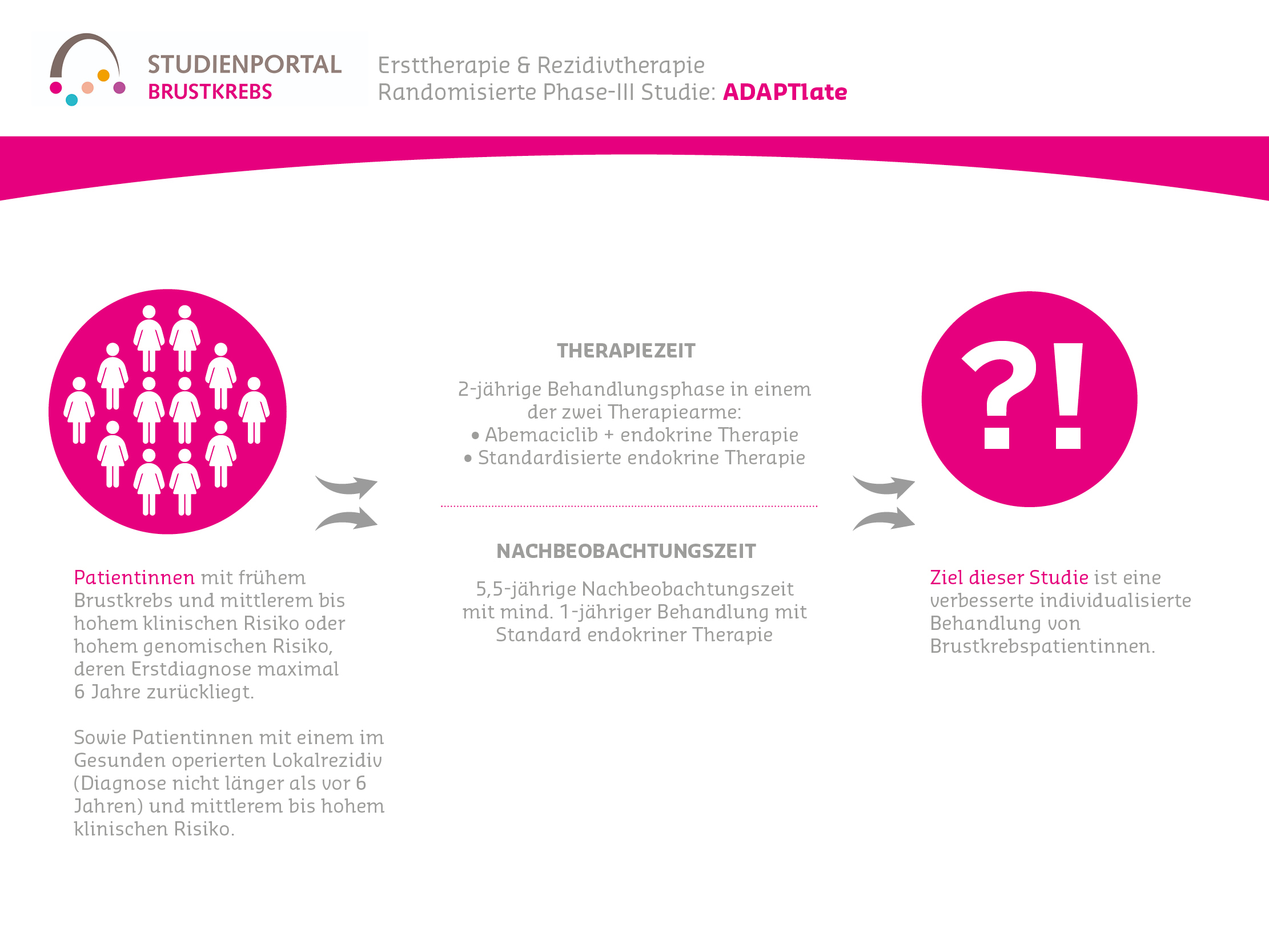
The ADAPTlate is a randomized, controlled, open-label, personalized Phase 3 therapy study adapted to dynamic tumor markers, targeting pre- and postmenopausal women with HR-positive and HER2-negative early-stage breast cancer who have a clinically or genomically high risk of late recurrence (late recurrence of the disease). This study compares the efficacy and safety of therapy with Abemaciclib plus standard endocrine therapy versus standard endocrine therapy alone after surgery.
Patients with intermediate clinical risk and unknown genomic risk can also be included and will receive genomic testing within the study. Furthermore, patients with isolated locoregional recurrence (re-occurrence of the tumor in the same breast or its vicinity) with a high-risk profile can participate in ADAPTlate.
What is being investigated in this study?
Patients with a high risk of recurrence are currently treated as standard with chemotherapy plus endocrine therapy (ET). However, this patient group cannot always optimally benefit from standard endocrine therapy. Furthermore, the tumor often develops resistance to ET at the time of recurrence. The ADAPTlate study aims to investigate whether patients derive additional benefit from treatment with Abemaciclib in combination with ET compared to ET alone.
Abemaciclib belongs to the so-called cyclin-dependent kinase inhibitors or CDK 4/6 inhibitors. Cyclin-dependent kinases are proteins that are significantly involved in cell cycle control and are often overactive in tumor cells. Inhibition of these proteins can lead to a reduction in tumor growth. Abemaciclib has been approved as a drug since September 2018 for women with hormone receptor-positive breast cancer that has metastasized or is locally advanced. In 2022, the combination therapy of Abemaciclib and ET was approved for women with HR-positive, HER2-negative, node-positive early-stage breast cancer with a high risk of recurrence.
What is the aim of the study?
The overarching goal of the study is to improve decision-making for individualized, tumor-specific treatment of early breast carcinomas. Specifically, the study examines the invasive disease-free survival of patients treated with Abemaciclib + ET compared to the control group treated with standard ET. Additionally, the study also investigates the quality of life of patients in both treatment arms.
How is the study conducted?
The study comprises two phases:
Phase 1:
2-year treatment phase in one of the two treatment arms
– Abemaciclib + endocrine therapy
– Standardized endocrine therapy according to clinical guidelines
Phase 2:
Follow-up phase of at least 1 year with standard ET for up to a maximum of 5.5 years
Are there risks?
You will be informed about possible risks or side effects associated with participation during an informational discussion.
Eligibility Criteria
Women aged 18 years or older can participate in this study, with:
- Primary early-stage breast cancer (without distant metastases) or
- locoregional recurrence (re-occurrence of the tumor in the same breast) with a high-risk profile
- Intermediate to high clinical or high genomic risk of breast cancer recurrence
- (Post)menopausal or premenopausal status
- Estrogen and/or progesterone hormone receptor positive
- HER2 receptor negative
- Initial diagnosis no more than 6 years ago
In addition, there are other criteria that must be met for study participation. Interested patients should speak with the investigators at a study center, who can assess whether this study is suitable for them.