PHERGain-2 Study
A PHASE 2 STUDY TO EVALUATE THE EFFICACY OF A CHEMOTHERAPY-FREE STRATEGY WITH SUBCUTANEOUS FIXED-DOSE TRASTUZUMAB, PERTUZUMAB, AND T-DM1 IN PATIENTS WITH PREVIOUSLY UNTREATED, HER2-POSITIVE EARLY BREAST CANCER
Recruitment for the study was completed in March 2024.
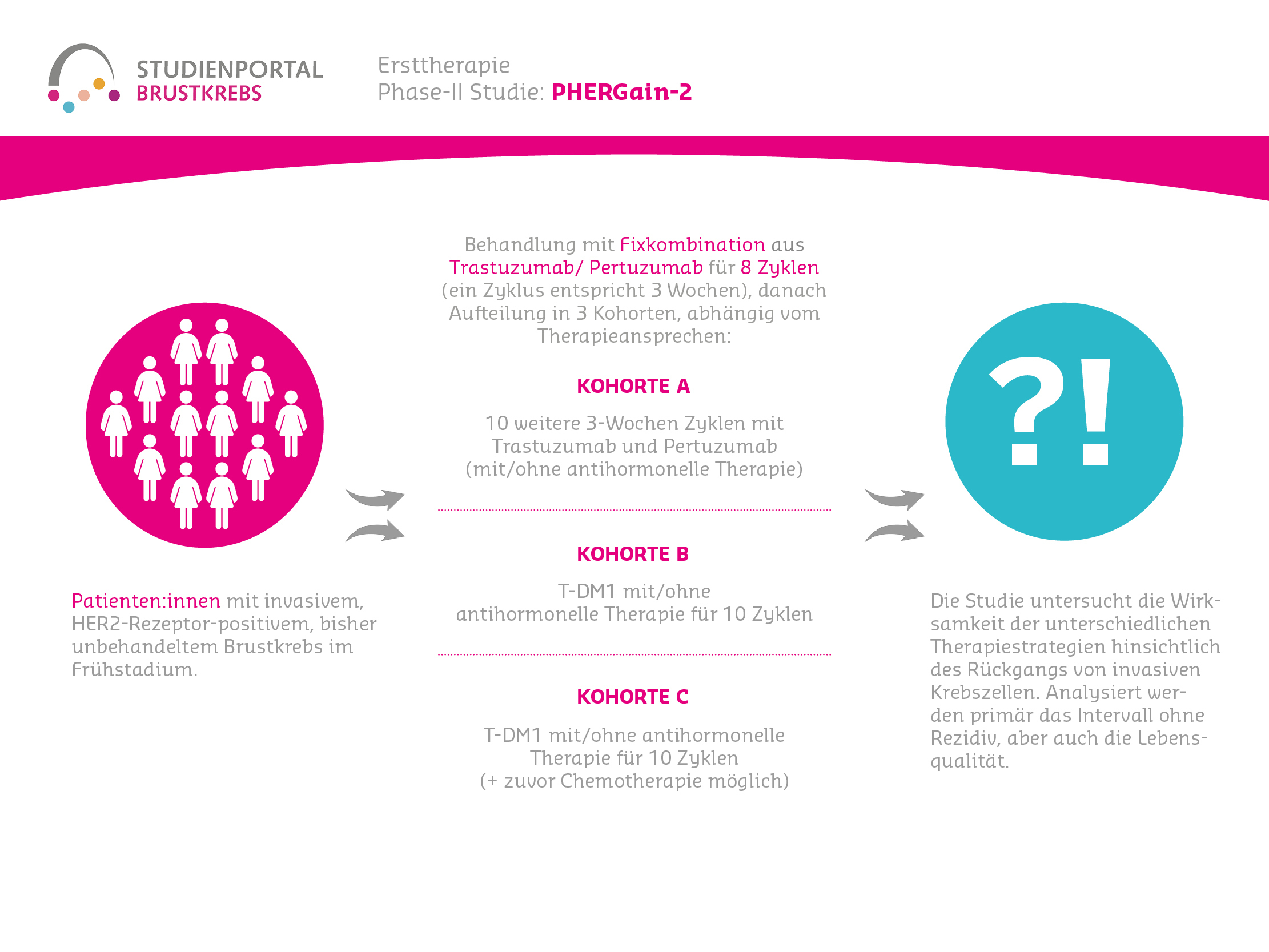
PHERGain-2 is a multicenter, unblinded, single-arm, single-stage Phase 2 study to evaluate the efficacy of a chemotherapy-free strategy, aiming for pathological complete response (pCR), with Trastuzumab and Pertuzumab (administered as a subcutaneous fixed-dose combination) and T-DM1 in patients with previously untreated HER2-positive early breast cancer.
What is being investigated in this study?
The study investigates the efficacy of a chemotherapy-free strategy that administers Trastuzumab and Pertuzumab as a fixed dose subcutaneously. Pertuzumab and Trastuzumab are antibodies that can bind to HER2 (a specific growth factor hormone receptor). HER2 causes tumor cells to grow faster and is present in large quantities in a quarter of breast cancer cases. By binding the antibodies to HER2, signals that stimulate tumor cell growth are prevented.
This is done in combination with T-DM1, Trastuzumab Emtansine, an antibody-drug conjugate where a drug is linked to an antibody. Antibodies are the “fighters” of the immune system, and in this context, they are also referred to as “armed” antibodies.
What is the goal of the study?
The study investigates the efficacy of this therapy strategy, with the goal being a “pathological complete response”, meaning the regression of invasive cancer cells. The primary analyses include recurrence-free interval, quality of life, and general health status.
What is the study procedure?
The study procedure has only one phase and one investigation arm. First, the HER2 status is examined and an MRI scan is performed. This is followed by the administration of Trastuzumab and Pertuzumab for 8 cycles (one cycle equals 3 weeks) as a fixed subcutaneous dose. After completion of the pre-operative (neoadjuvant) therapy, another MRI scan is performed, then the investigation arm is divided into 3 cohorts, depending on the pathological result:
- Cohort A: 10 additional 3-week cycles with Trastuzumab and Pertuzumab and, depending on HR status, endocrine therapy (ET, anti-hormonal therapy)
- Cohort B: T-DM1 with/without ET for 10 cycles
- Cohort C: T-DM1 with/without ET for 10 cycles, with the option of prior chemotherapy, at the discretion of the physicians
Are there risks?
You will be informed about possible risks or side effects associated with participation during an informational discussion.
Participation Requirements
Women and men aged 18 and older can participate in this study with the following criteria:
- Histologically confirmed, invasive breast cancer
- HER2 positive (Immunohistochemistry [IHC] 3+)
- Tumor size >5 to ≤ 25mm (≤ 30mm by MRI determination)
- Previously untreated
- No metastases
In addition, there are other criteria that must be met for participation in the study. Interested patients should speak with the investigators at a study center, who can assess whether this study is suitable for them.
This study is supported by: