SERENA-6 Study
Therapy study to evaluate the potential additional efficacy and safety of Camizestrant in the first-line treatment of patients with HER2-negative, HR-positive, ESR1-mutated breast cancer
Study recruitment was completed at the end of 2024.
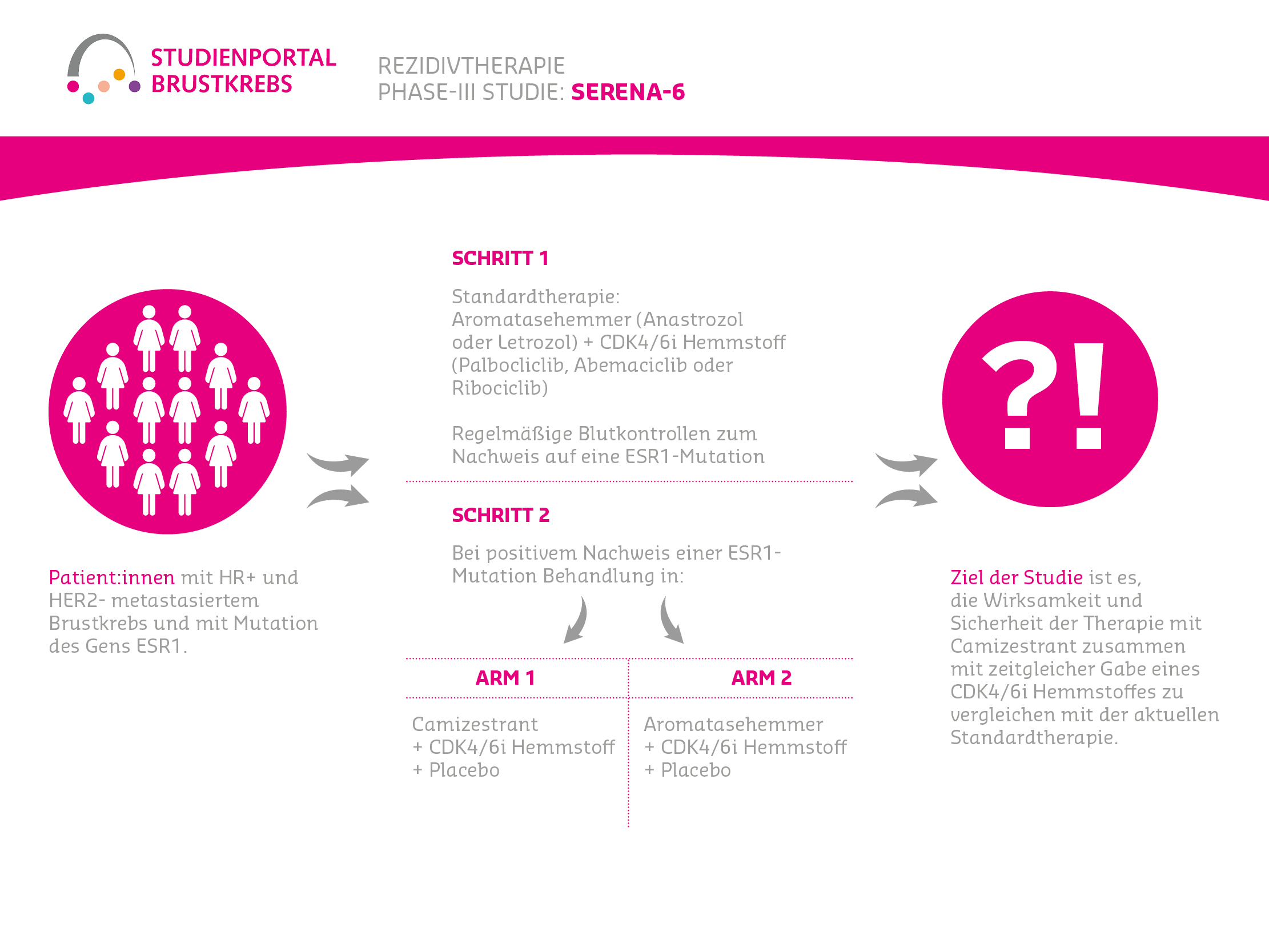
SERENA-6 is a double-blind (neither patient nor treating physicians know the treatment arm), randomized (random assignment to a treatment arm) Phase 3 study to evaluate the efficacy and safety of the drug Camizestrant compared to aromatase inhibitors (Letrozole or Anastrozole) in combination with Palbociclib or Abemaciclib or Ribociclib in patients with metastatic breast cancer that expresses hormone receptors (HR+) but not human epidermal growth factor receptor-2 (HER2-). The cancer must also show a detectable mutation in a specific gene (ESR1m) and the disease must not have progressed under first-line therapy.
What is being investigated in this study?
Hormone receptor-positive (HR+), human epidermal growth factor receptor-2-negative (HER2-) tumors currently account for more than two-thirds of all breast cancer cases. Current guidelines recommend a combined treatment for these tumors with endocrine therapy, e.g., an aromatase inhibitor and a cyclin-dependent kinase 4 and 6 (CDK4/6i) inhibitor, as first-line treatment for metastatic (spread to other organs) HR+/HER2- breast cancer. However, in many cases, drug resistance develops over time, which can lead to disease progression. Tumors with mutations in the estrogen receptor-alpha gene (ESR1m) are known to develop resistance to aromatase inhibitors and are also associated with more aggressive disease characteristics, including the development of visceral (affecting abdominal organs) metastases. Consequently, the expected treatment outcomes of subsequent therapies in patients with ESR1m-positive breast cancer are relatively poor, and new approaches are needed to improve treatment outcomes and prevent further disease progression.
Camizestrant is a next-generation oral (tablet) drug that destroys estrogen receptors in breast cancer cells (estrogen degrader). Tumors with hormone receptors can receive growth signals from naturally occurring hormones in the body (e.g., estrogen). Camizestrant is intended to disrupt this signaling and thus stop tumor growth. In previous studies, this drug was already effective against breast cancer (e.g., SERENA-1).
What is the goal of the study?
The primary goal of the study is to compare the efficacy of therapy with Camizestrant along with concurrent administration of a CDK4/6i inhibitor to the current standard therapy in patients with HER2-negative, HR-positive, ESR1-mutated breast cancer in first-line treatment (first treatment of newly diagnosed metastases). Additionally, the patients’ quality of life will be assessed using questionnaires.
How is the study conducted?
Step 1:
First-line breast cancer patients are treated according to current standards (aromatase inhibitor = Anastrozole or Letrozole + CDK4/6i = Palbociclib, Abemaciclib or Ribociclib) and regularly monitored (every 2-3 months) for an ESR1 mutation in cancer cells (via blood samples).
Step 2:
Patients whose tumor cells show an ESR1 mutation during one of these control examinations will be randomly assigned 1:1 to one of two treatment arms and will receive:
Arm A
Camizestrant + the previously received CDK4/6i inhibitor + a placebo as a substitute for the aromatase inhibitor
or
Arm B (Standard Therapy)
The previously received aromatase inhibitor + CDK4/6i inhibitor + a placebo as a substitute for the estrogen receptor degrader
Are there risks?
You will be informed about potential risks or side effects associated with participation during an informational discussion.
Eligibility Criteria
Men and women aged 18 and older can participate in this study, with:
- Hormone receptor-positive (HR+),
- HER2-negative,
- locally advanced (inoperable) or metastatic (spread) breast cancer
- Current first-line treatment with an aromatase inhibitor (Anastrozole or Letrozole) + CDK4/6i (Palbociclib, Abemaciclib or Ribociclib)
- ≥ 6 months without signs of disease progression
- For treatment in Step 2: Confirmed ESR1 mutation of the tumor
In addition, there are other criteria that must be met for participation in the study. Interested patients should speak with the investigators at a study center, who can assess whether this study is suitable for them.
Where can I participate in this study?
Further information on participating centers can be found here:
https://studienportal-brustkrebs.de/deutschlandkarte
This study is supported by: