DESTINY-Breast05 / GBG-103
Immunotherapy with antibody-drug conjugates in high-risk patients with early, HER2-positive breast cancer and residual tumor after completed chemotherapy, antibody treatment, and surgery
Study recruitment was completed in March 2024.
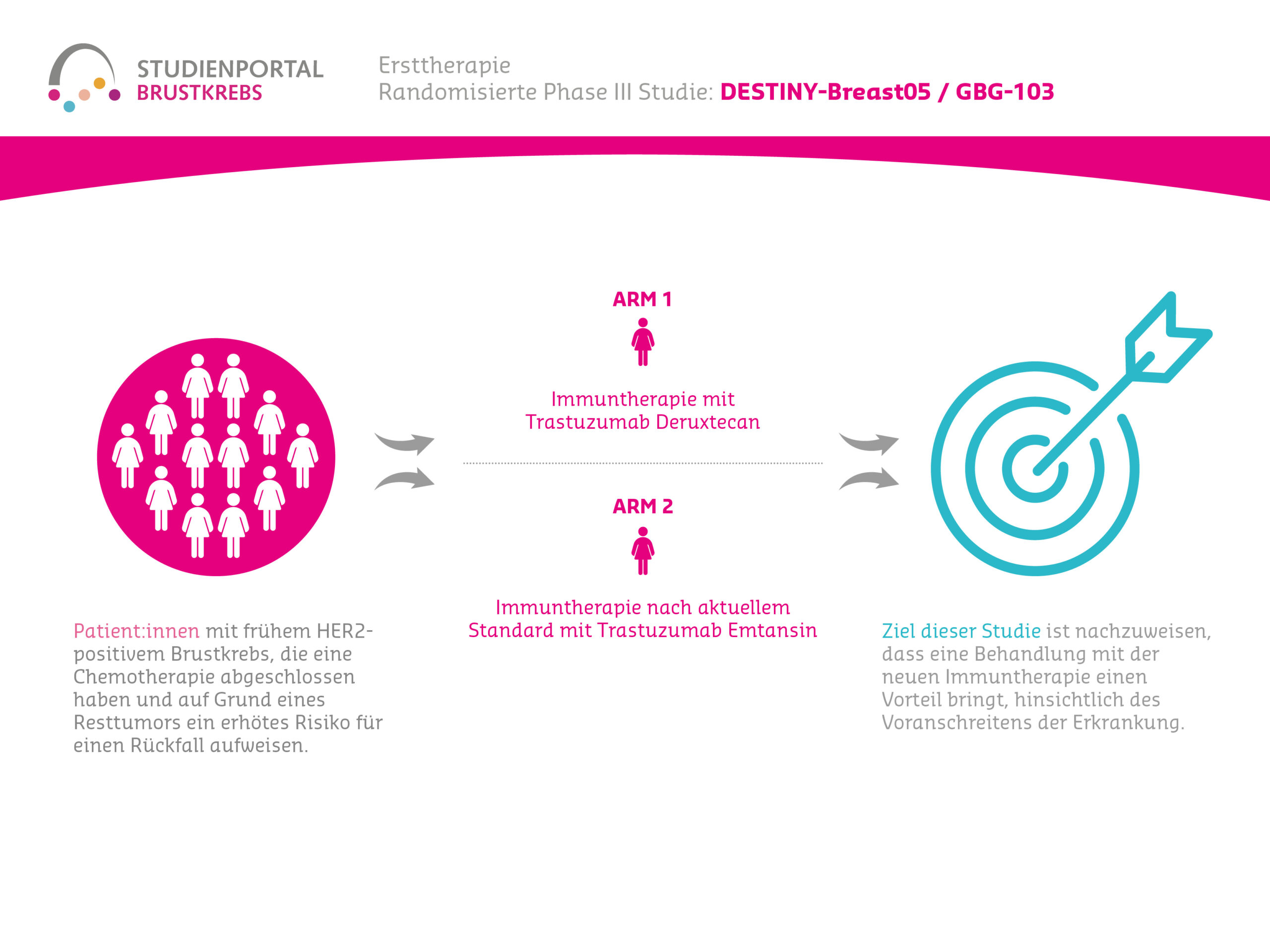
DESTINY-Breast05 is a randomized (treatment assignment for the specific patient is by chance), controlled, Phase 3 therapy study comparing two immunotherapies in patients with HER2-positive, early (patient has no metastases except for axillary lymph node metastases) breast cancer. For these patients, chemotherapy + anti-HER2 therapy (antibody administration, a form of immunotherapy) before surgery and the surgery itself have already been performed. Due to a residual tumor in the breast or lymph nodes in the armpits, these patients have a high risk of disease recurrence.
What is being investigated in this study?
A previous study (KATHERINE) with an antibody-drug conjugate (ADC, Trastuzumab Emtansine, T-DM1) was positive and confirmed the benefit of ADCs for patients after completed chemotherapy with antibody administration before surgery (neoadjuvant treatment) and confirmed residual tumor tissue after this treatment and surgery. An antibody-drug conjugate is a compound of a drug bound to an antibody. Most antibody-drug conjugates are chemoimmunoconjugates, meaning that a cell-damaging drug is linked to the antibodies. In this case, they are also referred to as armed antibodies. ADCs specifically bind to tumor cells and then release the drug, which can disrupt tumor growth.
Despite the positive KATHERINE study, there remains an unmet medical need in this patient group, as not all patients could be cured with the previous treatment. Therefore, the DESTINY-Breast05 study is planned for patients who underwent surgery after completed neoadjuvant therapy (chemotherapy + HER2-targeted therapy, Trastuzumab) and in whom a residual tumor was detected despite treatment (in the breast or axillary lymph nodes). The study aims to investigate whether the ADC Trastuzumab Deruxtecan (T-DXd) is more effective than the ADC Trastuzumab Emtansine (T-DM1) in these patients. Treatment with T-DM1 is currently the standard of care and serves as the control in this study.
What is the goal of the study?
The primary goal of this study is to demonstrate that treatment with Trastuzumab Deruxtecan has an advantage in terms of breast cancer progression or recurrence compared to Trastuzumab Emtansine in HER2-positive patients who have a residual tumor after completed chemotherapy and subsequent surgery. Furthermore, the study aims to investigate whether there is a correlation between the individual biomarker status of the tumor (HER2 and hormone receptor status) and the efficacy and/or safety of the treatment.
What is the study procedure?
Within the study, there are two treatment arms to which patients are randomly assigned in a 1:1 ratio:
Arm 1:
Trastuzumab Deruxtecan – intravenous injection every 3 weeks for approx. 12 months
Arm 2:
Trastuzumab Emtansine – intravenous injection every 3 weeks for approx. 12 months
Are there risks?
You will be informed about possible risks or side effects associated with participation during an informed consent discussion.
Eligibility requirements
Men and women aged 18 years and older can participate in this study, with:
- Initial diagnosis of breast cancer, without metastases except for axillary lymph node metastases
- HER2-receptor positive tumor
- Confirmed residual tumor in the breast or axillary lymph nodes
- Previously completed neoadjuvant chemotherapy and immunotherapy (antibody therapy) including Trastuzumab (anti-HER2 therapy)
In addition, there are other criteria that must be met for study participation. Interested patients should speak with the investigators at a study center, who can assess whether this study is suitable for them.
Further studies by the GBG Study Group can be found here.