GeparPiPPa / GBG-105
THERAPY STUDY TO EVALUATE THE POTENTIAL ADDITIONAL EFFICACY AND SAFETY OF INAVOLISIB IN THE PRE-OPERATIVE TREATMENT OF PATIENTS WITH HER2-POSITIVE, HR-POSITIVE, PIK3CA-MUTATED EARLY BREAST CANCER.
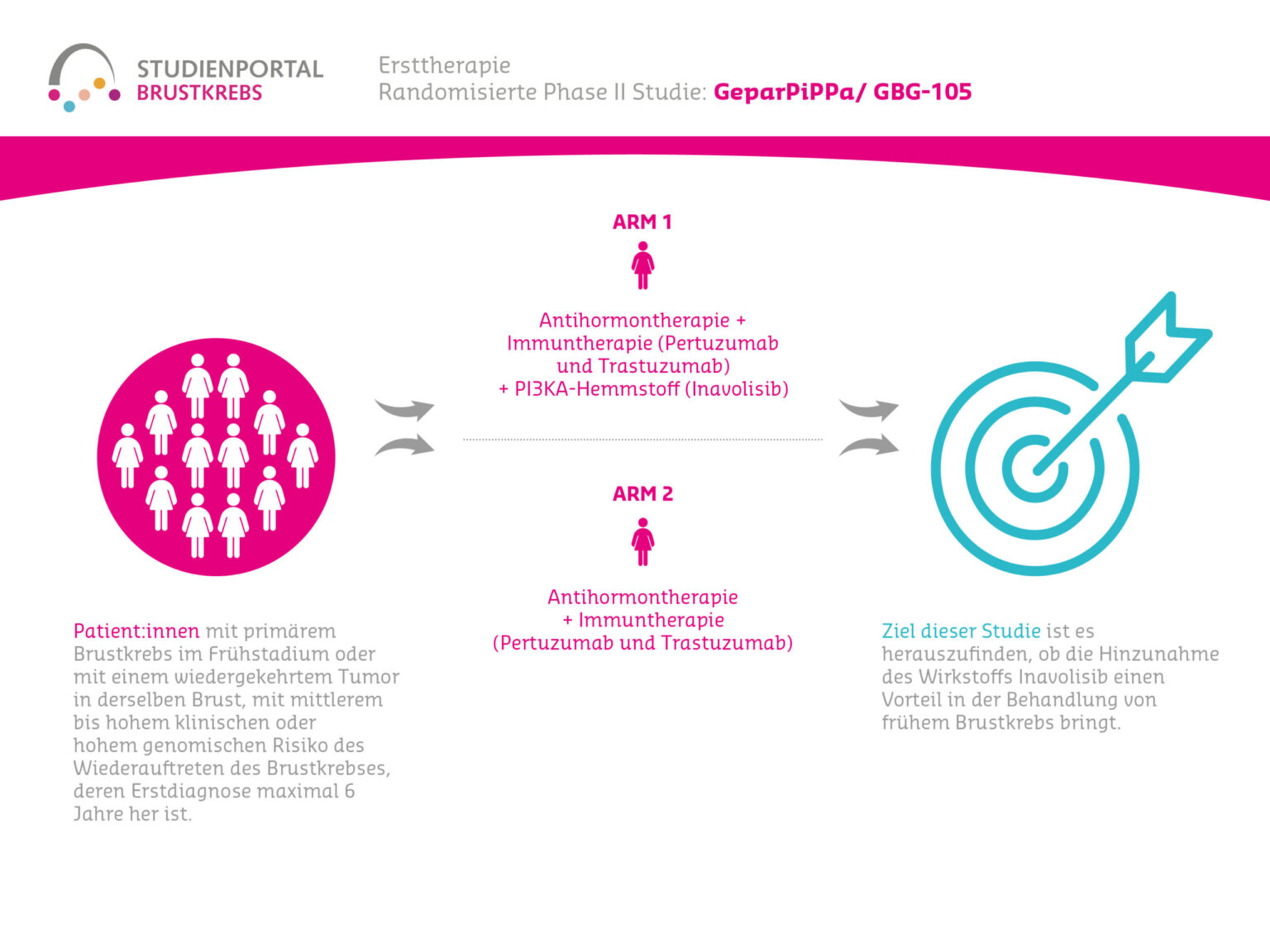
GeparPiPPa is a randomized (chance determines the treatment for each patient), non-blinded (doctor and patient know which medications are being used) Phase II study comparing pre-operative anti-hormone therapy in combination with the immunotherapeutics Trastuzumab, Pertuzumab with and without the PIK3 inhibitor Inavolisib in patients with HER2-positive, HR-positive, PIK3CA-mutated early (non-metastatic) breast cancer.
What is being investigated in this study?
The investigation of receptor status is a central aspect in the diagnosis of breast cancer. This status provides information about the presence of certain molecules in tumor cells or on their surface, which can serve as potential targets for drug treatments. One of these molecule groups is known as “HER2”. “HER2” stands for “Human Epidermal Growth Factor Receptor 2”. HER2 is a receptor on the cell surface of tumors that stimulates cell growth when activated. 20-30% of all patients with a high number of HER2 receptors (HER2 positive) on the tumor have a mutation in the PIK3CA gene. This gene is essential for the production of the enzyme PI3K, which is important for cell division, among other things. Tumors with PIK3CA mutations may have a poorer response to chemotherapy and anti-HER2 therapy. This is particularly true for breast cancer with positive hormone receptor expression (HR+). Inavolisib is an oral PI3KA inhibitor that is comparable to other drugs in the same class but is expected to have a better efficacy and tolerability profile.
What is the goal of the study?
The primary goal of the study is to compare the efficacy of therapy with Inavolisib along with concurrent endocrine therapy (anti-hormone therapy), Pertuzumab, and Trastuzumab versus endocrine therapy with Pertuzumab and Trastuzumab alone in patients with HER2-positive, HR-positive, PIK3CA-mutated early breast cancer. Additionally, the safety and tolerability of the treatment, as well as patient adherence, will be investigated.
How is the study conducted?
Breast cancer patients with a PIK3CA mutation will be randomly assigned 1:1 to one of two treatment arms and will receive:
Arm A
Anti-hormone therapy before surgery, in combination with immunotherapy (dual anti-HER2 blockade, consisting of Pertuzumab and Trastuzumab as an injection administered subcutaneously for 6 cycles (18 weeks)), combined with Inavolisib in tablet form for 6 cycles (18 weeks)
Arm B
Treatment regimen identical to Arm A, but without Inavolisib
Anti-hormone therapy consists of either Tamoxifen or an aromatase inhibitor (medication to inhibit estrogen production), possibly combined with a GnRH analog (medication to prevent ovulation) for premenopausal women and for all men. In both study arms, treatment will continue until surgery/biopsy is performed, or progressive, unacceptable side effects occur, or consent to the study is withdrawn.
Are there risks?
You will be informed about potential risks and side effects associated with participation during an informational discussion.
Participation Requirements
Men and women aged 18 and older can participate in this study, with:
- Untreated primary breast cancer (unilateral, no metastases)
- Breast tumor with a palpable diameter of ≥ 2 cm or an ultrasound diameter of ≥ 1 cm
- HER2 receptor and hormone receptor positive
- Confirmed PIK3CA mutation of the tumor
In addition, there are other criteria that must be met for participation in the study. Interested patients should speak with the investigators at a study center, who can assess whether this study is suitable for them.
Further studies by the GBG Study Group can be found here.