PROVIDENCE Study
A non-interventional study to collect clinical and quality of life data in patients with HER2+ or HER2-low inoperable or metastatic breast cancer who receive trastuzumab deruxtecan as second-line treatment (for HER2+) or trastuzumab deruxtecan in any line of therapy (for HER2-low) according to the professional information in routine clinical practice
The PROVIDENCE study is a purely observational study to evaluate the efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients with documented human epidermal growth factor receptor 2 (HER2)-positive or HER2-low inoperable or metastatic breast cancer who receive T-DXd according to the applicable product information in routine clinical practice in Germany. In addition, the quality of life reported by the participating patients in this patient collective will be investigated. Furthermore, patients will be informed about the use of the digital health application (DiGA).
What is the aim of the study?
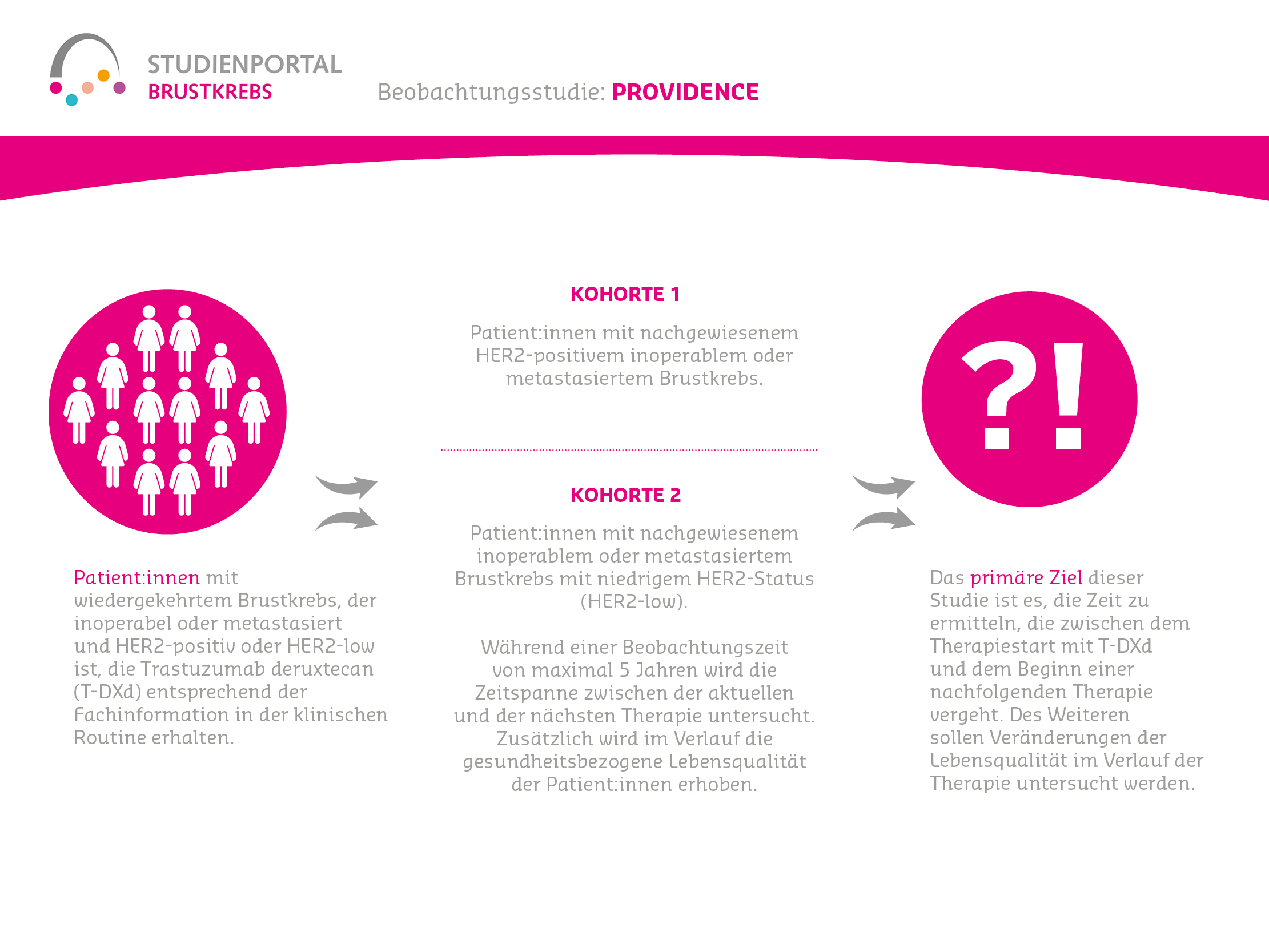
The primary objective of this study is to determine the time between the start of therapy with T-DXd and the start of a subsequent therapy. The observation period is a maximum of 5 years. Among other things, the health-related quality of life of the patients is also surveyed and their possible changes during the course of therapy are evaluated. In addition, the period in which the disease does not progress further is evaluated.
How is the study conducted?
Participants are divided into two cohorts based on their tumor biology (HER2 status):
Cohort 1 – Patients with confirmed HER2-positive inoperable or metastatic breast cancer who receive T-DXd according to routine clinical practice.
Cohort 2 – Patients with confirmed inoperable or metastatic breast cancer with low HER2 status (HER2-low) who are treated with T-DXd in routine clinical practice.
Are there any risks?
You will be informed about possible risks associated with participation during an explanatory discussion. Since this registry is purely a documentation of clinical routine without planned additional treatment, there is no additional health risk associated with participation.
Participation requirements
Women and men aged 18 years or older can participate in this study, with:
- recurrent breast cancer that is inoperable or metastatic
- HER2-positive or HER2-low
- Pre-treatment for HER2-positive: anti-HER2 targeted therapy
- Pre-treatment for HER2-low: chemotherapy
In addition, there are other criteria that must be met for participation in the study. Interested patients should speak with the study doctors at a participating center. They will check whether the PROVIDENCE study is suitable for you.
This study is supported by: