TROPION-Breast03 Study
Antibody-drug conjugate therapy with or without immunotherapy in patients with triple-negative and early breast cancer and residual tumor in the breast or axilla after neoadjuvant chemotherapy and completed surgery
Study recruitment was completed in November 2024.
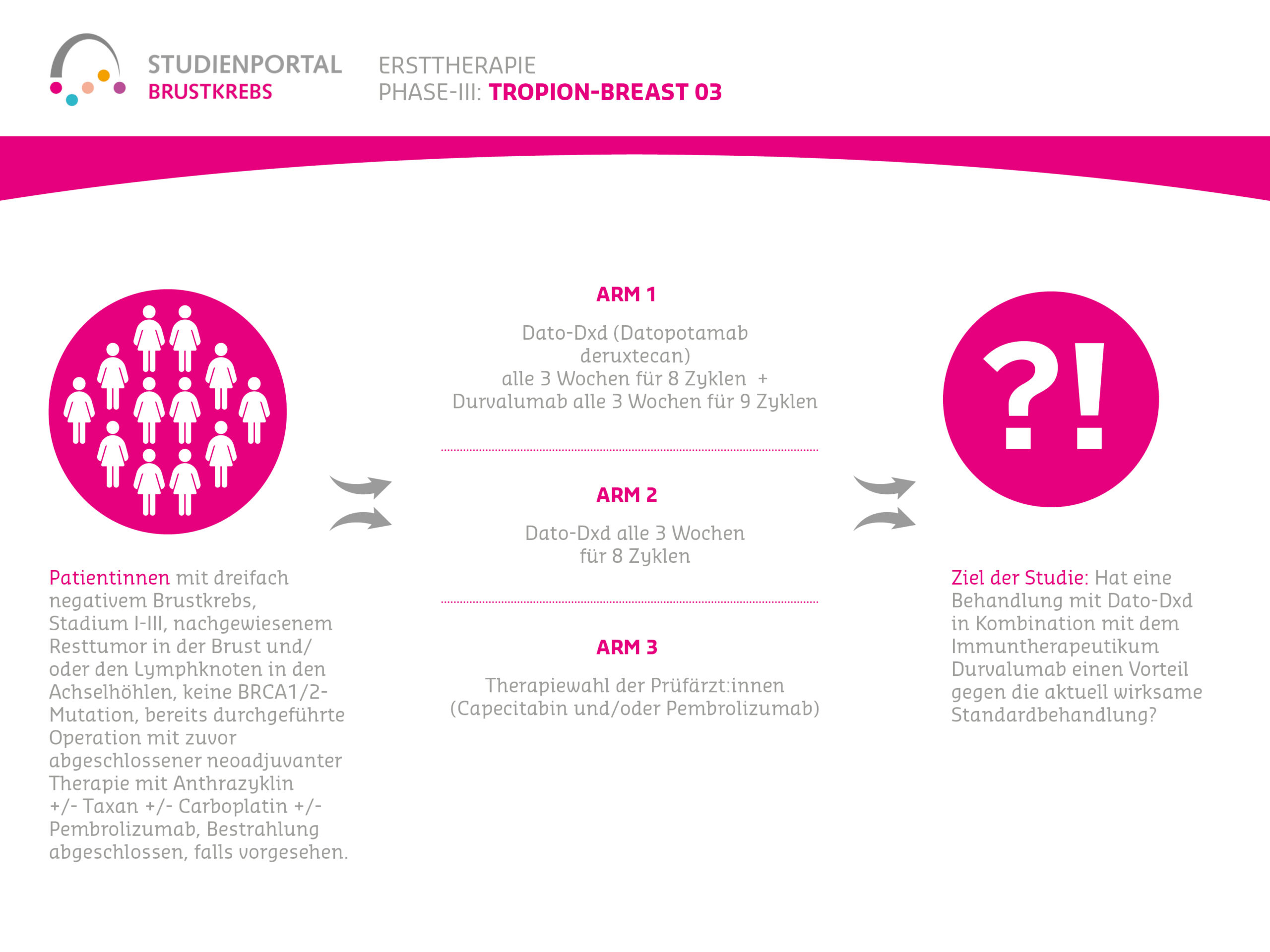
TROPION-Breast03 is an open-label, randomized (treatment assignment for the specific patient is by chance), controlled, Phase 3 therapeutic study. The study compares three different therapies and is aimed at patients with triple-negative (tumor cells have no estrogen, no progesterone, and no HER2 receptors), early (Stage I-III) breast cancer. In these patients, drug therapy was administered before surgery, and after surgery, residual tumor was found in the breast and/or axillary lymph nodes.
Triple-negative breast cancer is a type of breast cancer where the tumor cells have no estrogen or progesterone receptors in their nucleus and no HER2 receptors on their surface. Neoadjuvant chemotherapy is administered before the planned surgery. After neoadjuvant chemotherapy, the breast cancer and additional lymph nodes from the axilla are removed. If tumor cells are still detected in the removed tumor tissue, there is a high probability that tumor cells remain in the patient’s body and the breast cancer will recur. This necessitates further treatments.
What is being investigated in this study?
In patients with triple-negative breast cancer, the active substance Datopotamab deruxtecan (Dato-DXd) already showed efficacy against breast cancer in a previous study. Dato-DXd is an antibody-drug conjugate, i.e., a cancer drug linked to an antibody. Datopotamab deruxtecan binds to a protein called TROP2 located on cancer cells and is thereby taken up into the cancer cells, where the cancer drug is released and kills the cancer cells.
What is the aim of the study?
The TROPION-Breast03 study is designed for patients who underwent surgery after completing medicinal, neoadjuvant therapy and in whom residual tumor was detected despite treatment (in the breast or axillary lymph nodes). Patients with residual tumor generally have a higher risk of disease recurrence. The study investigates whether treatment with Dato-DXd in combination with the immunotherapy Durvalumab offers an advantage over the current, effective standard treatment (therapy choice by treating physicians).
Currently, there is no information available on whether all three treatments are equally effective or if one of the treatments is more effective than the others. To eliminate this uncertainty and to be able to provide patients with the most effective treatment in the future, studies like this TROPION-Breast03 study are being conducted.
What is the study procedure?
Within the scope of the study, there are three treatment options (“arms”) to which patients are randomly assigned in a 2:1:2 ratio:
Arm 1:
Datopotamab deruxtecan as an intravenous infusion every 3 weeks for 8 cycles (infusions) together with Durvalumab intravenous injection every 3 weeks for 9 cycles
Arm 2:
Datopotamab deruxtecan as an intravenous infusion every 3 weeks for 8 cycles
Arm 3:
Current therapy according to guidelines and at the discretion of treating physicians (Capecitabine and/or Pembrolizumab)
Are there any risks?
You will be informed about possible risks or side effects associated with participation during an informational discussion.
Eligibility criteria
Men and women aged 18 years or older can participate in this study, with:
- triple-negative breast cancer, Stage I-III
- Confirmed residual tumor in the breast or axillary lymph nodes
- No mutation in BRCA1/2 genes (detection in patient’s blood cells)
- Prior surgery and previously completed neoadjuvant therapy with anthracycline +/- taxane +/- carboplatin +/- pembrolizumab
- Previously completed radiation therapy (if applicable)
In addition, there are further criteria that must be met for participation in the study. Interested patients should speak with the investigators at a study center, who can check if this study is suitable for them.
Where can I participate in this study?
Further information on participating centers can be found here:
https://studienportal-brustkrebs.de/deutschlandkarte
This study is supported by: